Auditing the Informed Consent process
Prior to starting fertility treatment patients and their partners must complete a number of HFEA consent forms (Human Fertilisation and Embryology Authority).These forms record each parties consent to important medical and legal decisions required to comply with UK law governing fertility treatment.
Traditionally, taking the time to explain these forms in a clear and consistent manner has been problematic. Not least, because there is a lot of information for patients and partners to process and the decisions they are required to make have important and far reaching consequences for them and any child born.
Fertility Consent helps clinics in the informed consent process by providing an online patient education and consenting solution. At the same time, it helps clinics to manage and streamline their workflow; and our complete audit trail of all patient, partner, and clinic staff activities provides a permanent record of the entire consent process.
Explaining HFEA forms to fertility treatment patients
Fertility Consent has a complete library of short videos which explains each HFEA form in detail – clearly and consistently.
These videos can be watched at home at a time suitable to the patient and their partner. This allows individuals plenty of time to absorb information without any pressures, and helps to reduce the time spent in clinic.
Patients and their partners can watch and re-watch the videos as often as they wish, and ask their clinic staff any questions before completing their consent forms online.
Sample video explainer for HFEA CD form
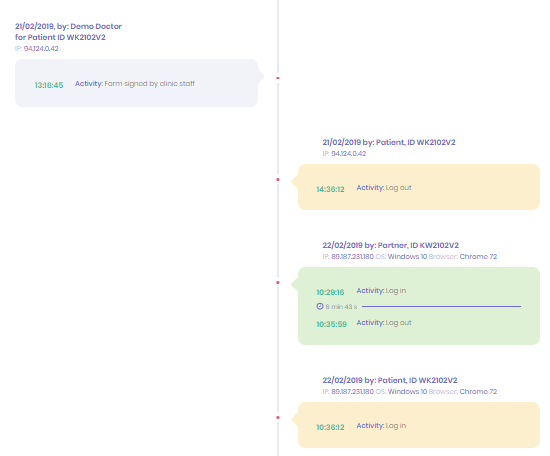
Fertility Consent Audit Trail
When it comes to having a detailed audit trail of each informed consent journey, Fertility Consent is unique.
From the time that a patient and their partner (if they have one) contacts a clinic to book an appointment, all the way through to the beginning their treatment Fertility Consent records each step in detail, including: the videos watched and acknowledged; information read and consent forms completed and signed.
This means that instead of relying on verbal confirmation that information has been received and understood, clinics now have a complete documented record that can be exported into the patient’s record.
The activities of clinic staff are also recorded as part of the audit trail, this means that any internal or external review of the informed consent process can ensure standard operating procedures are followed on a case by case basis.
HFEA Form and Video Library
Fertility Consent manages and maintains a library of all the current versions of the HFEA forms and provides a detailed video explainer of each one.
HFEA CD form: Your consent to disclosing identifying information
Who should fill in the HFEA CD form?
Fill in this form if:
- you or your partner are receiving fertility treatment
- you are donating eggs, sperm or embryos, or
- you are storing eggs, sperm or embryos for your or your partner’s future treatment.
If you are a donor, any consent given in this form will not affect your legal rights and responsibilities.
Your information will only be disclosed to the parties you agree to on this form.
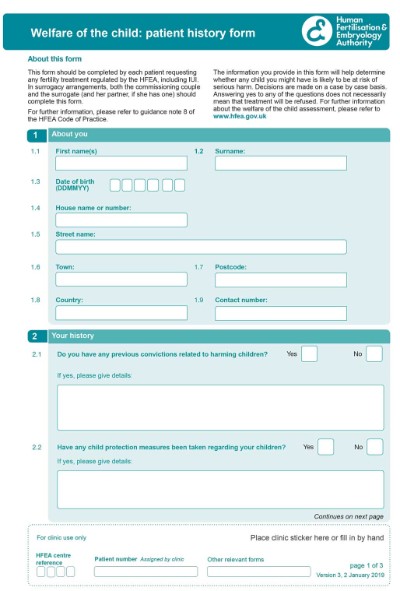
HFEA Welfare of the Child form:
Patient history form
Who should fill in the HFEA WoC form?
This form should be completed by each patient requesting any fertility treatment regulated by the HFEA, including IUI.
In surrogacy arrangements, both the commissioning couple and the surrogate (and her partner, if she has one) should
complete this form.
The information you provide in this form will help determine whether any child you might have is likely to be at risk of serious harm. Decisions are made on a case by case basis.
HFEA WT form: Women’s consent to treatment and storage form (IVF and ICSI)
Who should fill in the HFEA WT form?
Fill in this form if you are a woman and you are having fertility treatment using embryos created outside the body (in vitro) with your eggs.
This may be in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI).
HFEA MT form: Men’s consent to treatment and storage form (IVF and ICSI)
Who should fill in the HFEA MT form?
Fill in this form if you are a man and your partner is having fertility treatment using embryos created outside the body (in vitro) with your sperm.
This may be in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI).
HFEA GS form: Your consent to the storage of your eggs or sperm
Who should fill in the HFEA GS form?
Fill in this form if you want to store your eggs or sperm.
By law (the Human Fertilisation and Embryology Act 1990 (as amended)), you need to give your written consent if you want your eggs or sperm to be stored.
You must also state in writing how long you consent to your eggs or sperm remaining in storage.
This form allows you to consent to storage. You will need to complete an additional form if you want to use your eggs or sperm for treatment.
HFEA PP form: Your consent to being the legal parent
Who should fill in the HFEA PP form?
Fill in this form if you are:
- not married or in a civil partnership, and
- your partner is receiving treatment using donor sperm, or embryos created outside the body (in vitro) using donor sperm, and
- you wish to be the legal parent of any child born from your partner’s treatment.
HFEA WGI form: Your consent to the use of your eggs in GIFT
Who should fill in the HFEA WGI form?
Fill in this form if you are a woman and you are having gamete intra-fallopian transfer (GIFT) using your eggs.
By law (the Human Fertilisation and Embryology Act 1990 (as amended)), you need to give your written consent if you want your eggs to be used in fertility treatment.
If you are planning to store any eggs that are left after your treatment, you are legally required to record what you would like to happen to them if you were to die or lose the ability to decide for yourself (i.e. become mentally incapacitated).
While this is perhaps not something you have considered, your clinic needs to know this so that they only allow your eggs to be used according to your wishes.
HFEA MGI form: Your consent to the use of your sperm in artificial insemination
Who should fill in the HFEA MGI form?
Fill in this form if you are a man and your partner is having artificial insemination. This may be intrauterine insemination (IUI) or gamete intrafallopian transfer (GIFT) using your sperm.
By law (the Human Fertilisation and Embryology Act 1990 (as amended)), you need to give your written consent if you want your sperm to be used in fertility treatment.
If you are planning to store any sperm that are left after your treatment, you are legally required to record what you would like to happen to your sperm if you were to die or lose the ability to decide for yourself (i.e. become mentally incapacitated).
While this is perhaps not something you have considered, your clinic needs to know this so that they only allow your sperm to be used according to your wishes.
HFEA WC form:
Withdrawing your consent
Who should fill in the HFEA WC form?
Fill in this form if you wish to withdraw your consent to:
- the use or storage of your eggs, sperm or embryos, or
- being the legal parent of any child born as a result of your partner’s treatment – you can only do this if donor sperm or embryos are being used in your partner’s treatment and you are not married to/in a civil partnership with them, or
- your partner being the legal parent of any child born as a result of your treatment – you can only do this if donor sperm or embryos are being used in your treatment and you are not married to, or in a civil partnership with, your partner.